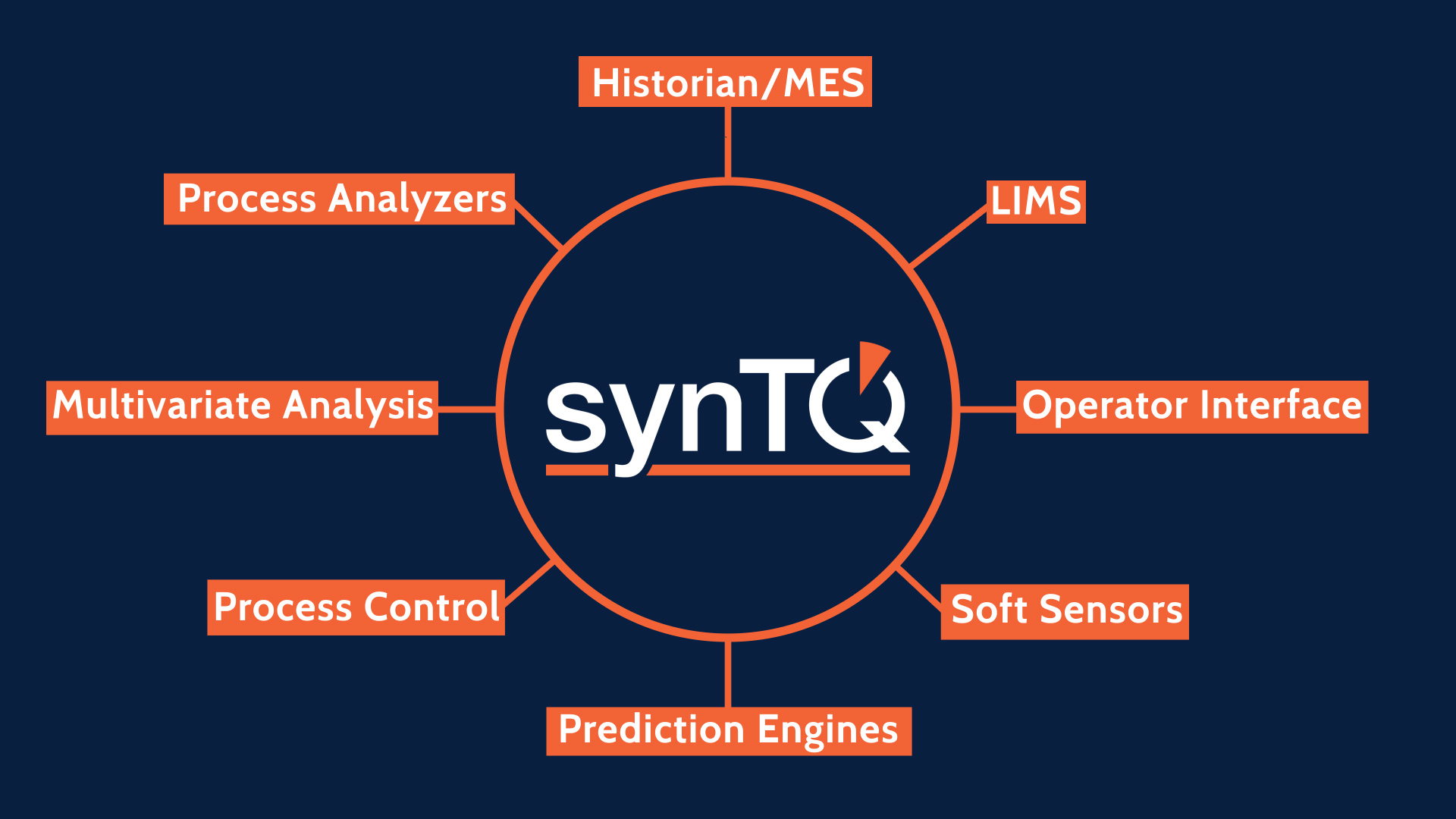
About synTQ
Achieve the huge cost saving benefits of PAT enabled manufacture via the most effective, easiest to use real-time PAT knowledge manager on the market…
“There is no doubt that Process Analytical Technology (PAT) is the key emergent process technology that is allowing the pharmaceutical industry to make the step change from the stricture of slow, inefficient and cumbersome batch production techniques to fully integrated regulatory compliant continuous production techniques .”
Martin Gadsby, Director Optimal
Join the Revolution…
The synTQ PAT Knowledge Management Software Suite has been quickly adopted by several of the top 10 global drug producers to effectively implement PAT, providing universal hardware and software system integration via effective real-time data recording and data management from the development lab, through to full-scale production.
PAT (Process Analytical Technology)
PAT can simultaneously reduce manufacturing costs, improve product quality and dramatically reduce the time to market for new compounds. Continuous manufacturing and
To find out more, read our article on synTQ-Lite!
Instrumentation Management
The use of complex instrumentation (NIR, Raman, Particle sizing etc.) offers the potential to reveal in real time those Critical Quality Attributes of your product. To access this information however, the correct configuration and calibration/referencing status of the instruments is vital as without these being robustly controlled and recorded then all your gathered data will be invalidated. synTQ controls all possible attributes of your instrument as well as controlling the operation of the instruments and of course gathering and storing all of the output spectral data. synTQ has a large and ever expanding library of instrument adaptors enabling bidirectional communication with them, and if we don’t happen to have an adaptor for your specific instrument then we will undertake to write one for you. Thus synTQ assures the complete integrity of all data associated with all of your instruments.
Control
The FDA’s guidance document clearly states that PAT can only be fully realised with a robust control element. synTQ enables the implementation of this control element by using a sophisticated and graphically based PAT Method creation facility, known as ‘Orchestrations’.This methodology allows the CQAs that have been generated by the
Data Storage & Reporting
PAT is highly
Vendor Neutral Connectivity
All synTQ Editions are designed to be vendor neutral. Provided that the device requiring connection has a communications interface and a known protocol, synTQ can connect to it. You are not forced to use any specific vendor’s control system, instrument or indeed Multivariate Analysis (MVA) package – you are free to select these, creating a “best of breed” solution. As your systems evolve, synTQ’s multi-vendor connectivity capability allows you to easily add new instruments, control systems or indeed MVA packages with the minimum of fuss and validation, this allowing you to take full advantage of the latest and optimum technologies.
Analysis & Real Time Prediction
Process model building with synTQ becomes a simple, quick and accurate process. synTQ communicates with most of the commonly used Multivariate Analysis (MVA) packages enabling the easy association and export of automatically acquired and Laboratory analysis data. This export is carried out within the synTQ environment and as such is fully 21CFR Part 11 compliant. The resulting process models are kept within the synTQ environment and stored in the database. When running in real time synTQ passes the acquired data from multiple instruments along with any univariate data to the MVA prediction engine which will have been pre-loaded with the correct MVA model. This then calculates the required Critical Quality Attributes of your product and stores them in the database and makes them available for any other required action.
Continuous Improvement
One of the major changes which will need to be embraced following the advent of PAT is that of continuous improvement. Processes which were once ‘set in stone’ will now be constantly monitored, and when appropriate changes introduced in a controlled way to improve process capability. synTQ is designed to facilitate the implementation of these changes in a straightforward and robust way. Version control of all facets of data storage ensures that the historical development of a process can be fully traced from inception and the enhanced graphing facilitates the continual enhancement of Process Understanding.
PAT Consultancy & Training
PAT is a major change for the pharmaceutical industry, and embarking on a PAT implementation can be a very daunting task. The deployment of PAT demands the application of multiple skill sets, and if you don’t yet have all the skills or resources to move forward with your implementation then Optimal can offer a wide range of specialised consultancy services including PAT training to fill the gaps.

Configuration & Installation
As well as being the developers of synTQ, we at Optimal have been designing and installing bespoke automation systems for pharmaceutical, chemical and food industry clients for over 25 years. We are therefore well placed to offer a full configuration, installation, commissioning and validation service. This can cover all aspects of a PAT system as well as the more traditional PLC/SCADA/DCS/ MES automation requirements.
Global Supply and Support
For many years, we at Optimal have been providing support for critical manufacturing systems all around the world, and to further enhance this we now provide a 24/7/365 product support service called synTQ Assured. If required, synTQ Assured application support can also be provided either directly from us or in collaboration with your local
approved synTQ provider. From a supply perspective synTQ can be supplied either directly from us,from Emerson Process Management else via one of our continually expanding approved synTQ Partners.
